Answer:
The correct answer is option c.
Step-by-step explanation:
Molecularity is defined as number of atoms , ions or molecules that must collide with one another so as to result in a chemical reaction.It is simply the sum of molecules of different reactants as represented in balanced chemical equation.
Reaction taking place in one step are said to be elementary reaction.
Reaction taking place in more than one step are said to be complex reaction.
A mechanism for the decomposition of ozone is given as:
1)
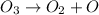
Molecularity for the first elementary reaction is 1. This because single ozone molecule is present.
2)
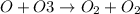
Molecularity for the second elementary reaction is 2. This because single ozone molecule reacts with 1 oxygen atom.