Answer:
V₂=153.84 m L
Step-by-step explanation:
Given that
Initial volume ,V₁ = 200 m L
Initial pressure ,P₁= 500 torr
Final pressure P₂ = 650 torr
Lets take the final volume =V₂
Given that process occurs at constant temperature and we know that for constant temperature process
P₁ V₁ = P₂ V₂
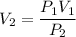
Now by putting the values
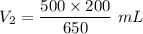
V₂=153.84 m L
Therefore the new volume of the gas will be 153.8 4mL.