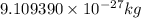Step-by-step explanation:
Every atom consists of three sub-atomic particles which are protons, neutrons and electrons.
Inside the nucleus of an atom, there will be only protons and neutrons. Whereas electrons always revolve around the nucleus of an atom.
Protons have a positive charge, neutrons have no charge and electrons have a negative charge.
Mass of these subatomic particles are as follows.
Proton :
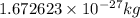
Neutron :
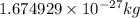
Electron :