Answer:
d)50.0 mL
Step-by-step explanation:
At equivalence point ,
Moles of
 = Moles of NaOH
= Moles of NaOH
Considering :-
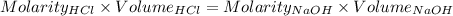
Given that:
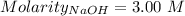
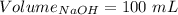
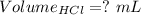
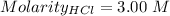
So,
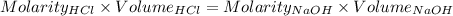
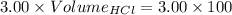
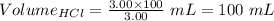
The volume of HCl at equivalence point is - 100 mL
More addition of the acid forms a acidic solution. Thus, d)50.0 mL is the correct option because all other options are greater or equal to 100 which leads to acidic or neutral solution respectively.