Answer : The expression for reaction quotient will be :
![Q_c=([Zn^(2+)])/([Ag^(+)]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/h1aausbicx4zu2snndjel4n0aqqqsyi0mu.png)
Explanation :
Reaction quotient (Qc) : It is defined as the measurement of the relative amounts of products and reactants present during a reaction at a particular time.
The given redox reaction is :
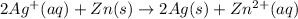
In this expression, only gaseous or aqueous states are includes and pure liquid or solid states are omitted.
The expression for reaction quotient will be :
![Q_c=([Zn^(2+)])/([Ag^(+)]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/h1aausbicx4zu2snndjel4n0aqqqsyi0mu.png)