Answer: The ionic reaction is written below.
Step-by-step explanation:
Complete ionic equation is defined as the equation in which chemical species are written in the form of ions. All the spectator ions are present in this equation.
The chemical equation for the reaction of copper with silver nitrate is given as:
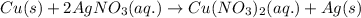
Ionic form of the above equation follows:
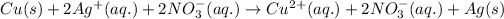
This equation is known as single displacement reaction in which more reactive element displaces a less reactive element from its chemical reaction.
Hence, the ionic reaction is written above.