Answer:
1. Look at the attached image
2. Look at the attached image
3. Mass Number
4. Proton =14
5. Proton = electron = 82 , Neutron = 125
6. Atomic number = 28
7. neutron = 197
8. More by neutron = 2
9. Charge on Al = Zero(Neutral)
10 .Valance electron = 6
11.charge on S = -2
12. Valence electron = 1
13. charge on Na = +1
Step-by-step explanation:
Atomic Number = Total number of protons present in the nucleus of the atom.
Atomic number = proton
Mass Number = Total number of proton and neutron present in the nucleus of the atom.
Mass number = number of proton + neutron
Symbolic representation :
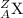
A = mass number = proton + neutron
Z = atomic number = proton
X = element
Charged Species = Ions = These are formed by loss of gain of electron by the atom .
Negative charge = Gain electron
Positive Charge = Loss of electron
Que 1.
In the table :
Symbol = From periodic table you have to know the symbols of element and their atomic number.
Atomic number = Periodic table
Proton = Atomic number : Proton and atomic number are same
Electron count :
For neutral element : Number of proton = Number of electron
E.g
Electron in Fe = 26 = proton number
Electron in K = 19 = proton number
For Negative Charged specied : Charge + Proton
For Positive Charged specied : Proton - Charge
For I-
Charge = 1
Electron = Proton + charge = 53 + 1 = 54
Que 2.
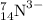
Proton = Z = 7
Neutron = A - Z = 14 - 7 = 7
electron = proton + charge = 7 + 3 = 10
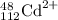
Proton = Z = 48
Neutron = A - Z = 112 - 48 = 64
electron = proton + charge = 48 -2 = 46
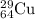
Proton = Z = 29
Neutron = A - Z = 64 -29 =35
electron = proton = 35 (no charge)
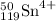
Proton = Z = 50
Neutron = A - Z = 119-50 = 69
electron = proton = 50 - 4 = 46
Que 3.
The Number 79 - In Se-79 represent the (Mass Number)
Q4
Protons present in Si - 28 = 14 ( atomic nuber of Si = 14)
Q5.
The Number of subatomic particles in 207 Pb =
Proton = 82
neutron =207-82 =125
Electron = 82
Q6.
Atomic number for an element with 31 neutron and mass number 59 = 28
Q7. The mass number for gold having 118 neuton is = 197
Q8 . more neutron are there in Br-80 than Br-78 (80 - 78) = 2
Q9 . Charge on Al atom = Zero
Q 10. Valance electron in S = 6
Valancy = total electron present in outermost shell
Its atomic number = 16
Electronic Configuration = 2 + 8 + 6 (=16)
It has 6 electron in outermost shell
Q11. Charge on S = -2
To complete its (8 electron in outermost shell) octet ,S gains 2 electron . Hence it get -2 charge
Q12. Valance electron in Na = 1
Its atomic number = 11
Electronic Configuration = 2 + 8 + 1 (=11)
It has 1 electron in outermost shell
Q13. Charge on Na = +1
To complete its (8 electron in outermost shell) octet ,Na loses 1 electron . Hence it get +1 charge