Answer:
The percent composition of each element are :
1. Th(SO4)2 :
Th = 54.704% , S = 15.119% , O = 30.1759 %
2.Ca3(PO4)2
Ca = 38.76% , P = 19.97% , O = 41.265%
3.C6H12O6
C = 40.0% , H = 6.72% , O = 53.29%
Step-by-step explanation:
Formula calculate the percent composition

Molar mass of Th(SO4)2 = Mass of Th + 2(mass of S) + 8(mass of O)
= 232.03 + 2(32.06) + 8(16)
=232.03 +64.12+128
= 424.16

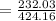
= 54.704%

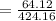
= 15.119%

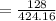
= 30.17%
2.Ca3(PO4)2
Molar mass of Ca3(PO4)2 = 3(Mass of Ca) + 2(mass of P) + 8(mass of O)
= 3(40) + 2(30 .9) + 8(16)
= 120 + 61.8 + 128
=310.176

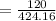
38.76%

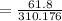
= 19.97%

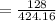
= 41.26 %
3.
C6H12O6
Molar mass of C6H12O6 = 6(Mass of C) + 12(mass of O) + 6(mass of O)
= 6(12) + 12(1) + 6(15.99)
=180

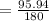
= 53.28%


= 40%

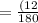
= 6.713%