Answer:
Yes , 5 mole of Iron Oxide has mass of 798.5 g
Step-by-step explanation:
Formula of iron oxide:
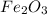
Atomic mass of Fe = 55.84 amu
Atomic mass of O = 15.99 amu
Molar mass of Fe2O3 = 2(atomic mass of Fe) + 3(atomic mass of O)
= 2(55.84) + 3(15.99)
=111.68 + 47.97
= 159.69 g/mol
Molar mass always equal to 1 mole of the substance.
1 mole = 159.69 g
5 mole =
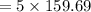
= 798.45 g