Answer:
The answer is 2.09 moles
Step-by-step explanation:
Avogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023 * 10²³ particles per mole. The Avogadro number applies to any substance.
Then you can apply a rule of three as follows: if 6.023*10²³ particles are contained in 1 mole, 1.26*10²⁴ particles in how many moles are they?
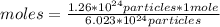
moles= 2.09