Step-by-step explanation:
Molality is the number of moles present in kilogram of solution. Mathematically, expression for molality is as follows.
Molality =
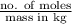
As we increase the temperature of a solution then there occurs no change in its mass as well as no change in its number of moles. As a result, there will occur no change in molality of the solution.
Molarity is the number of moles present in liter of solution. So, with change in temperature there occurs change in volume of the solution. Hence, molarity will also change.
Normality is no. of gram equivalents per liter of solution. As normality is also dependent on volume and when there occurs a change in temperature then volume of solution also changes. As a result, normality will also change with change in temperature.