The question is incomplete, here is the complete question:
15.00 mL of a 0.127 M
 solution are mixed with 25.60 mL of a 0.137 M
solution are mixed with 25.60 mL of a 0.137 M
 solution. What is the molar concentration of ions in the resulting solution?
solution. What is the molar concentration of ions in the resulting solution?
Answer: The concentration of calcium and chloride ions in the resulting solution are 0.133 M and 0.266 M respectively.
Step-by-step explanation:
To calculate the molarity of the solution after mixing 2 solutions, we use the equation:

where,
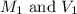 are the molarity and volume of the first
are the molarity and volume of the first
 solution
solution
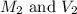 are the molarity and volume of the second
are the molarity and volume of the second
 solution
solution
We are given:
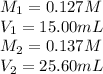
Putting all the values in above equation, we get:

The chemical equation for the ionization of calcium chloride follows:
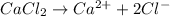
1 mole of calcium chloride produces 1 mole of calcium ions and 2 moles of chloride ions
So, concentration of calcium ion = 0.133 M
Concentration of chloride ions =
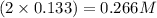
Hence, the concentration of calcium and chloride ions in the resulting solution are 0.133 M and 0.266 M respectively.