Step-by-step explanation:
Organic compounds are defined as the compounds which contain carbon as their main element. For example,
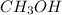 is an organic compound.
is an organic compound.
Generally, organic compounds are non-polar in nature and due to the presence of covalent bonding organic compounds have low melting point.
As compound A melts at
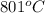 and is soluble in water. This means it is an ionic compound as it has high melting point and it is also polar in nature.
and is soluble in water. This means it is an ionic compound as it has high melting point and it is also polar in nature.
Whereas compound B melts at
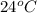 and is insoluble in water. This means that this compound has covalent bonding and it is also non-polar in nature . Hence, it is more likely to be organic in nature.
and is insoluble in water. This means that this compound has covalent bonding and it is also non-polar in nature . Hence, it is more likely to be organic in nature.
Thus, we can conclude that compound B is more likely to be an organic
compound.