Step-by-step explanation:
Chemical formula of hexane is
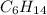 and chemical formula of octane is
and chemical formula of octane is
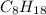 . Molecular mass of hexane is 86.18 g/mol and molecular mass of 114.23 g/mol.
. Molecular mass of hexane is 86.18 g/mol and molecular mass of 114.23 g/mol.
Smaller is the molecular mass of a substance then less dispersion forces will be present in it. As a result, it will have less strong bonding between its combining atoms. Therefore, it will have high volatility.
Therefore, hexane having small molecular mass will be more volatile in nature as compared to octane.
Therefore, in the given situation when labels from both the bottles disappear then liquid which will evaporate readily into the atmosphere will be hexane and another one will be octane.