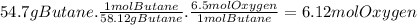Answer:
6.12 mol
Step-by-step explanation:
Let's consider the following balanced equation.
C₄H₁₀ + 6.5 O₂ → 4 CO₂ + 5 H₂O
We can establish the following relations.
- The molar mass of butane is 58.12 g/mol.
- The molar ratio of butane to oxygen is 1:6.5.
The moles of oxygen needed to react with 54.7 g of butane are: