Step-by-step explanation:
Noble gas configuration is defined as the configuration which contains completely filled orbitals.
For example, oxygen atom when gain two electrons then it forms oxygen ion (
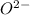 ).
).
Atomic number of oxygen atom is 8 and so, its number of electrons will also be 8. But when it gain two electrons then it has total 10 electrons. Hence, electronic configuration of
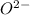 is as follows.
is as follows.
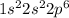
Since, there are completely filled orbitals in an
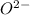 ions. Therefore, it means this ion has a noble-gas configuration.
ions. Therefore, it means this ion has a noble-gas configuration.
Thus, we can conclude that any specie which shows completely filled orbitals will have noble-gas configuration.