Answer: The equivalent mass of the acid is 83.16 grams
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
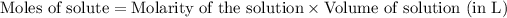
Molarity of
 solution = 0.1165 M
solution = 0.1165 M
Volume of
 solution = 24.68 mL = 0.02468 L
solution = 24.68 mL = 0.02468 L
Putting values in equation 1, we get:
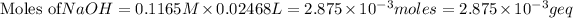
( as acidity of NaOH is 1)
For end point: gram equivalents of acid = gram equivalents of base =
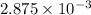
Mass of acid=
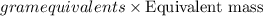
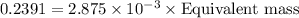
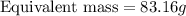
Thus equivalent mass of the acid is 83.16 grams