Answer:
![[H_3O^+]=6.8*10^(-22)](https://img.qammunity.org/2021/formulas/chemistry/college/ww2pbglw98t5jhhdg1alg2x8i4halwgw1g.png)
Step-by-step explanation:
For a solution with
![[Pb^(+2)]=1*10^(-6)](https://img.qammunity.org/2021/formulas/chemistry/college/jz87qrkos2mmkjuibrouf5feqdot2cp3cs.png) :
:
![Kps=[Pb^(+2)]*[S^(-2)]=3.4*10^(-28)](https://img.qammunity.org/2021/formulas/chemistry/college/92hv8bhh69g44mbgnpz4a3u0w1dqf7e26m.png)
![1*10^(-6)*[S^(-2)]=3.4*10^(-28)](https://img.qammunity.org/2021/formulas/chemistry/college/grms5qil7u6657ueh5ryojark31excpgda.png)
![[S^(-2)]=3.4*10^(-22)](https://img.qammunity.org/2021/formulas/chemistry/college/7hp0qfeia5w6xsxihkwyitb0tqhxz51rcn.png)
For the iron ions:
![Kps=[0.365]*[S^(-2)]=3.7*10^(-19)](https://img.qammunity.org/2021/formulas/chemistry/college/3lo3eobw66upd2201gxgx2kd1p809wc7xi.png)
![[S^(-2)]=1.01*10^(-18)](https://img.qammunity.org/2021/formulas/chemistry/college/q9zsh1nlo11uy6c2mh94hqp6ugzshvpu79.png)
Given that the
![[S^(-2)]](https://img.qammunity.org/2021/formulas/chemistry/college/j9idlloj2fr23we9o10m6fjcauy6smty20.png) concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate.
concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate.
The concentration of
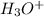
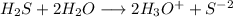
![[H_3O^+]=2*[S^(-2)]=6.8*10^(-22)](https://img.qammunity.org/2021/formulas/chemistry/college/zl12nwn5ljxq8mafhoss35dlm3pomdq9w6.png)