Given question is incomplete. The complete question is as follows.
Pentaborane (
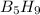 ) is a colorless highly reactive liquid that will burst into flames when exposed to oxygen.the reaction is:
) is a colorless highly reactive liquid that will burst into flames when exposed to oxygen.the reaction is:
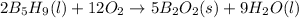
Calculate the kilojoules of heat released per gram of the compound reacted with oxygen.the standard enthalpy of formation
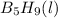 ,
,
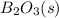 , and
, and
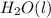 are 73.2, -1271.94, and -285.83 kJ/mol, respectively.
are 73.2, -1271.94, and -285.83 kJ/mol, respectively.
Step-by-step explanation:
As the given reaction is as follows.
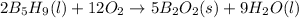
Therefore, formula to calculate the heat energy released is as follows.
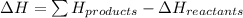
Hence, putting the given values into the above formula is as follows.
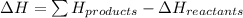
=
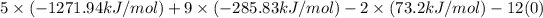
= -9078.59 kJ/mol
Since, 2 moles of Pentaborane reacts with oxygen. Therefore, heat of reaction for 2 moles of Pentaborane is calculated as follows.
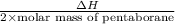
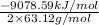
= -71.915 kJ/g
Thus, we can conclude that heat released per gram of the compound reacted with oxygen is 71.915 kJ/g.