Step-by-step explanation:
It is known that the relation between pH and
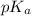 is as follows.
is as follows.
pH =
![pK_(a) + log ([salt])/([acid])](https://img.qammunity.org/2021/formulas/chemistry/college/5n90csyrjf636z261lnwricnu5e37tae9h.png)
and,
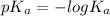
Hence, first we will calculate the value of
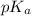 as follows.
as follows.
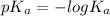
=
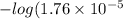
= 4.75
Now, we will calculate the value of pH as follows.
pH =
![pK_(a) + log \frac{[\text{sodium acetate}]}{\text{acetic acid}}](https://img.qammunity.org/2021/formulas/chemistry/college/rqinqyd9vz6t3pvbwrsp467dym8e5pt9hg.png)
=
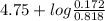
= 4.75 + (-0.677)
= 4.07
Therefore, we can conclude that the pH of given solution is 4.07.