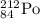Answer: The original element is
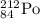
Step-by-step explanation:
Alpha decay is defined as the process in which alpha particle is emitted. In this process, a heavier nuclei decays into a lighter nuclei. The alpha particle released carries a charge of +2 units.
The released alpha particle is also known as helium nucleus.
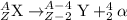
For the given alpha decay process of an isotope:
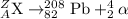
To calculate A:
Total mass on reactant side = total mass on product side
A = 208 + 4
A = 212
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
Z = 82 + 2
Z = 84
The isotopic symbol of unknown element is
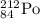
Hence, the original element is