Answer:
Moles of potassium chloride = 0.000672 moles
Step-by-step explanation:
Considering:
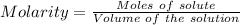
Or,
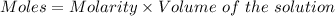
Given :
For potassium chloride :
Molarity = 0.12 M
Volume = 5.6 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 5.6×10⁻³ L
Thus, moles of potassium chloride :
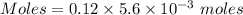
Moles of potassium chloride = 0.000672 moles