The given question is incorrect. The correct question is as follows.
If 20.0 g of
 and 4.4 g of
and 4.4 g of
 are placed in a 5.00 L container at
are placed in a 5.00 L container at
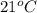 , what is the pressure of this mixture of gases?
, what is the pressure of this mixture of gases?
Step-by-step explanation:
As we know that number of moles equal to the mass of substance divided by its molar mass.
Mathematically, No. of moles =
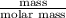
Hence, we will calculate the moles of oxygen as follows.
No. of moles =
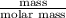
Moles of
 =
=
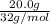
= 0.625 moles
Now, moles of
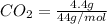
= 0.1 moles
Therefore, total number of moles present are as follows.
Total moles = moles of
 + moles of
+ moles of

= 0.625 + 0.1
= 0.725 moles
And, total temperature will be:
T = (21 + 273) K = 294 K
According to ideal gas equation,
PV = nRT
Now, putting the given values into the above formula as follows.
P =
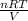
=
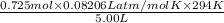
=
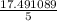 atm
atm
= 3.498 atm
or, = 3.50 atm (approx)
Therefore, we can conclude that the pressure of this mixture of gases is 3.50 atm.