Answer:
20.10 pounds of carbon dioxide are produced from burning one gallon of gasoline.
Step-by-step explanation:
Volume of the gasoline = V = 1 gal = 3785.41 mL
Density of the gasoline = d = 0.78 g/ml
Mass of gasoline = m = ?
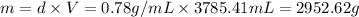
Moles of gasoline =
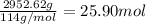
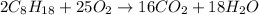
According to reaction, 2 moles of octane gives 16 moles of carbon dioxide gas.
Then 25.90 moles of octane will give:
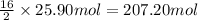
Mass of 207.20 moles of carbon dioxide gas:
= 207.20 mol × 44 g/mol = 9,116.86 g
1 pound = 453.592 grams
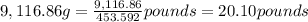
20.10 pounds of carbon dioxide are produced from burning one gallon of gasoline.