Answer:
1.22 L of carbon dioxide gas
Step-by-step explanation:
The reaction that takes place is:
- CaCO₃ + HCl → CaCl₂ + CO₂ + H₂O
First we determine which reactant is limiting:
- Calcium carbonate ⇒ 10.0 g CaCO₃ ÷ 100 g/mol = 0.10 mol CaCO₃
- Hydrochloric acid ⇒ 0.100 L * 0.50 M = 0.05 mol HCl
So HCl is the limiting reactant.
Now we calculate the moles of CO₂ produced:
- 0.05 mol HCl *
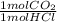 = 0.05 mol CO₂
= 0.05 mol CO₂
Finally we use PV=nRT to calculate the volume:
- T = 25 °C ⇒ 25 + 273.16 = 298.16 K
1 atm * V = 0.05 mol * 0.082 atm·L·mol⁻¹·K⁻¹ * 298.16 K