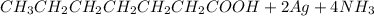Answer:
![CH_3 CH_2 CH_2 CH_2 CH_2 CH_2 CHO + 2[Ag(NH_3)_2]+ 3 H_2 O](https://img.qammunity.org/2021/formulas/chemistry/college/6n589ep97qm33527by0s1mwug6lwlzpesw.png) =
=
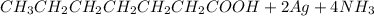
Step-by-step explanation:
Tollens test is carried out to perceive difference between aldehydes and ketones on the basis of their capability to oxidized easily.
when Tollens react with aldehyde (heptanal) , a silver mirror is form on inner side of container.
The reaction between tollens and heptanal is given as
![CH_3 CH_2 CH_2 CH_2 CH_2 CH_2 CHO + 2[Ag(NH_3)_2]+ 3 H_2 O](https://img.qammunity.org/2021/formulas/chemistry/college/6n589ep97qm33527by0s1mwug6lwlzpesw.png) =
=