Answer: 42 hours
Step-by-step explanation:
Moles of electron = 1 mole
According to mole concept:
1 mole of an atom contains
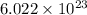 number of particles.
number of particles.
We know that:
Charge on 1 electron =
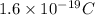
Charge on 1 mole of electrons =
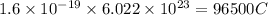
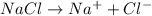
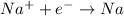
According to stoichiometry:
1 mole of electrons deposit = 23 g of sodium
i.e 96500 C of electricity deposit = 23 g of sodium
Thus 23 g of sodium is deposited by = 96500 C of electricity
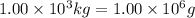 of sodium is deposited by =
of sodium is deposited by =
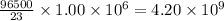 C of electricity
C of electricity
To calculate the time required, we use the equation:
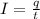
where,
I = current passed =
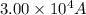
q = total charge =
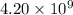
t = time in secods = ?
Putting values in above equation, we get:
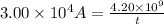
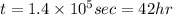
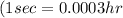
Thus 42 hours are required.