Answer:
0.4383 g
Step-by-step explanation:
Molality is defined as the moles of the solute present in 1 kg of the solvent.
It is represented by 'm'.
Thus,
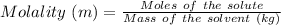
Given that:
Mass of solvent, water = 150 g = 0.15 kg ( 1 g = 0.001 g )
Molality = 0.050 m
So,
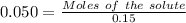
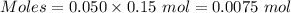
Molar mass of NaCl = 58.44 g/mol
Mass = Moles*Molar mass =
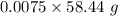 = 0.4383 g
= 0.4383 g