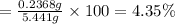Answer:
Percent acetic acid in the vinegar is 4.35%.
Step-by-step explanation:
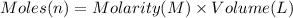
Moles of NaOH = n
Volume of NaOH solution = 30.84 mL = 0.03084 L
Molarity of the NaOH = 0.128 M
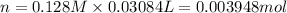
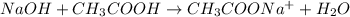
According to reaction ,1 mole of NaOH reacts with 1 mol of acetic acid.
Then ,0.03948 mol of NaIOH will recat with:
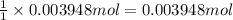 of acetic acid.
of acetic acid.
Mass of 0.03948 moles of acetic acid = 0.003948 mol × 60 g/mol = 0.2368 g
Mass of vinegar solution = 5.441 g
Percent acetic acid in the vinegar :