Answer:
5.85 grams of NaCl should be added to 250 grams of water to make a 0.40 m solution.
Step-by-step explanation:
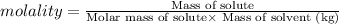
Mass of NaCl =x
Molar mass of NaCl = 58.5 g/mol
Molality of the solution = 0.40 m
Mass of the solvent = 250 g = 0.250 kg (1g = 0.001 kg)
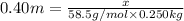
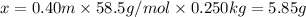
5.85 grams of NaCl should be added to 250 grams of water to make a 0.40 m solution.