Step-by-step explanation:
Atomic number of magnesium is 12 and its electronic distribution is 2, 8, 2. Whereas the atomic number of oxygen is 8 and its electronic distribution is 2, 6.
Magnesium in order to attain stability needs to lose two electrons and hence it forms
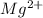 ion. Similarly, oxygen atom on gaining two electrons to complete its octet will form
ion. Similarly, oxygen atom on gaining two electrons to complete its octet will form
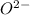 ion.
ion.
Therefore, magnesium and oxygen when chemically combine together then magnesium will donate its valence electrons to the oxygen atom leading to the formation of magnesium oxide.
Chemical formula of magnesium oxide is MgO. Hence, it means the value of x in
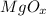 is 1.
is 1.