Step-by-step explanation:
It is known that molarity is the number of moles present in a liter of solution.
Mathematically, Molarity =
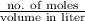
Hence, calculate the molarity of given solution as follows.
Molarity of citric acid =
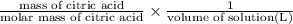
=
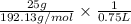
= 0.173 M
As citric acid is a triprotic acid so, upon dissociation it gives three hydrogen ions.
Normality = Molarity × no. of hydrogen or hydroxide ions
= 0.173 × 3
= 0.519 N
Thus, we can conclude that molarity of given solution is 0.173 and its normality is 0.519 N.