Answer:
The reagent is HCl.
Step-by-step explanation:
To separate Cu²⁺ from Ag⁺ in solution, we can use HCl since it forms an insoluble chloride, AgCl, while the Cu²⁺ ions remain in solution. This can be represented by the following equation:
Ag⁺(aq) + Cu²⁺(aq) + Cl⁻(aq) ⇄ AgCl(s) + Cu²⁺(aq)
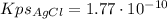
I hope it helps you!