Answer:
X = 0.005%
Step-by-step explanation:
The concentration of X remaining in the water phase can be calculated using the next equation:
![[X]_(i) = ((V_(aq))/(V_(or)KD + V_(aq)))^(i) \cdot [X_(0)]](https://img.qammunity.org/2021/formulas/chemistry/college/v787027qdlz97s7xxve21lbrqdp5c03zo6.png)
Where:
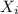 : is the concentration of A remaining in aqueous solution
: is the concentration of A remaining in aqueous solution
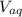 : is the volume of water
: is the volume of water
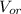 : is the volume of the organic solvent
: is the volume of the organic solvent
KD: is the distribution constant of X between water and the organic solvent
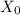 : is the original concentration of X
: is the original concentration of X
i: is the number of extractions with the organic solvent
![[X]_(i) = ((50 mL)/(10 mL \cdot 13.5 + 50 mL))^(4) \cdot 0.01 M = 5.34 \cdot 10^(-5) M](https://img.qammunity.org/2021/formulas/chemistry/college/vm0ekav0y0xg5w6wbiswxelaw1d50qvejr.png)
The percent of X remaining in the water phase is:
![[X]_(i) = 5.34 \cdot 10^(-5) \cdot 100 \% = 0.005 \%](https://img.qammunity.org/2021/formulas/chemistry/college/j59akpb3606lqjs53hywvpw4n79q82sucj.png)
I hope it helps you!