Answer:
we will use the Clausius-Clapeyron equation to estimate the vapour pressures of the boiling ethanol at sea level pressure of 760mmHg:
ln (P2/P1) =
/(T1)](https://img.qammunity.org/2021/formulas/chemistry/college/7cbf077hli16j1anpxa8w9gcm352qxqpn6.png) -
-
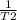 )
)
where
P1 and P2 are the vapour pressures at temperatures T1 and T2
Δ vapH = the enthalpy of vaporization of the ETHANOL
R = the Universal Gas Constant
In this problem,
P 1 = 100 mmHg
; T 1 = 34.7 °C = 307.07 K
P 2 = 760mmHg
T 2 =T⁻²=?
Δ vap H = 38.6 kJ/mol
R = 0.008314 kJ⋅K -1 mol -1
ln ( 760/10)=(0.00325 - T⁻²) (38.6kJ⋅mol-1 /0.008314 )
0.0004368=(0.00325 - T⁻²)
T⁻²=0.002813
T² = 355.47K