Answer: The amount of ice melted is 50.3 grams.
Step-by-step explanation:
To calculate the heat released by water, we use the equation:
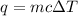
where,
q = heat released
m = mass of water = 100 g
c = specific heat capacity of water = 4.186 J/g.°C
 = change in temperature =
= change in temperature =
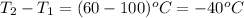
Putting values in above equation, we get:
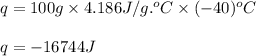
The amount of heat released by water will be absorbed by ice.
So, amount of heat absorbed by ice = -q = -(-16744) J = 16744 J
To calculate the enthalpy change of the reaction, we use the equation:
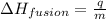
where,
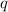 = amount of heat absorbed = 16744 J
= amount of heat absorbed = 16744 J
m = mass of ice melted = ?
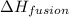 = heat of fusion = 333 J/g
= heat of fusion = 333 J/g
Putting values in above equation, we get:
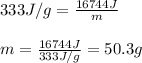
Hence, the amount of ice melted will be 50.3 g