To develop this problem, we will apply Einstein's relationship which is in charge of the work done with the kinetic energy of the body versus the total energy of the system.
The energy can be calculated as
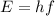
Here,
h = Planck's Constant
f = Frequency
Our values are given as,

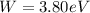
Therefore the Energy is
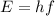
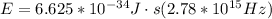
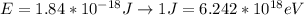
Then,
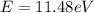
Applying the Einstein Relation we have that
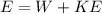
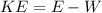
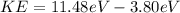
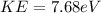
Therefore the maximum kinetic energy for an electron dislodged fromthe surface by the radiation is 7.68eV