Answer:
a. 0.01 C
b. dissipated to outside environment
Step-by-step explanation:
Let the specific heat of copper be 0.3846 kJ/kg-K or 384.6 J/kg-C
(a)The original kinetic energy of the block is:
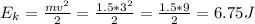
As 85% of this kinetic energy is converted to block internal heat energy, with specific heat we can calculate the rise in temperature:
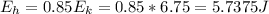
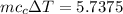
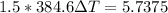
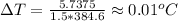
(b) the remaining 15% energy would probably be dissipated to outside environment as heat energy