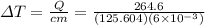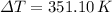Answer:
The increase in temperature of the bullet is 351.1 kelvin
Step-by-step explanation:
First, we should find the kinetic energy of the bullet is:
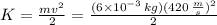
with m the mass and v the velocity.
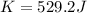
Now we know that half of the kinetic energy of the bullet is transformed into internal energy, by second's law of thermodynamics that means heat (Q) to raise bullet temperature (T), so:
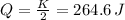
To know what the increase in temperature is, we should use specific heat of lead:
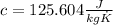
The equation that relates specific heat, change in temperature and mass is:
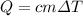
solving for
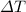 :
: