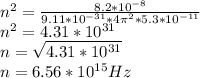Answer:
n=6.56×10¹⁵Hz
Step-by-step explanation:
Given Data
Mass=9.1×10⁻³¹ kg
Radius distance=5.3×10⁻¹¹m
Electric Force=8.2×10⁻⁸N
To find
Revolutions per second
Solution
Let F be the force of attraction
let n be the number of revolutions per sec made by the electron around the nucleus then the centripetal force is given by
F=mω²r......................where ω=2π n
F=m4π²n²r...............eq(i)
as the values given where
Mass=9.1×10⁻³¹ kg
Radius distance=5.3×10⁻¹¹m
Electric Force=8.2×10⁻⁸N
we have to find n from eq(i)
n²=F/(m4π²r)