Answer:
From Maxwell-Boltzmann distribution of molecules and their speeds,
average speed, Vavg = √(
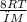 )
)
and Root mean speed, Vrms = √(
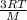 )
)
Given Vavg2 = 5Vrms1
Vavg2 = √(
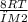 ) ................ eqn 1
) ................ eqn 1
Vrms1 = √(
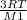 ) --------------eqn 2
) --------------eqn 2
To find m1/m2, we divide eqn 1 by eqn 2,
we have, Vavg2/Vrms1 = √(
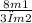 )
)
since Vavg2 = 5Vrms1, it becomes, 5 = √(
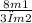 )
)
Therfore, m1/m2 = 25 * 3π/8 = 29.45
the mass ratio m1/m2 = 29.45.