Final answer:
As per the values, 0.561 moles of sodium oxide should be produced with 6.50 g of sodium reacting with oxygen gas.
Step-by-step explanation:
To calculate the number of moles of sodium oxide produced, we first need to write the balanced chemical equation for the reaction between sodium and oxygen. The equation is:

From the equation, we can see that 4 moles of sodium react with 1 mole of oxygen gas to produce 2 moles of sodium oxide. So, the mole ratio is: 4 moles Na : 1 mole O2 : 2 moles Na2O
To find the number of moles of sodium oxide produced, we use the given mass of sodium and its molar mass:
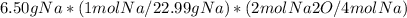
= 0.561 mol Na2O
Therefore, 0.561 moles of sodium oxide should be produced with 6.50 g of sodium reacting with oxygen gas.