Answer:
The answer to your question is 0.45 moles
Step-by-step explanation:
Balanced equation
NH₄NO₃ ⇒ N₂O + 2H₂O
2 ----------- N ----------- 2
4 ------------ H ----------- 4
3 ------------ O ----------- 3
From the balanced equation we know that 1 mol of NH₄NO₃ produces 2 moles of water, so we use proportions to find the amount of NH₄NO₃ required to produce 0.9 moles of water.
1 mol of NH₄NO₃ ------------- 2 moles of water
? -------------- 0.9 moles of water
Use cross multiplication
x =
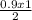
Simplification
x = 0.9 / 2
Result
x = 0.45 moles of NH₄NO₃