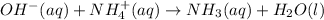Step-by-step explanation:
(a) When aqueous solution of hydrochloric and ammonia chemically react together then it results in the formation of ammonium chloride.
The reaction equation for this reaction is as follows.
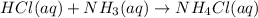
Now, ioni equation for this reaction is as follows.
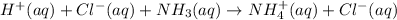
Cancelling the spectator ions, the net ionic reaction equation is as follows.
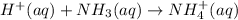
(b) When sodium hydroxide chemically reacts with ammonium chloride then it forms ammonia, water and sodium chloride as follows.
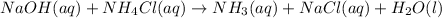
Now, the total ionic equation will be as follows.
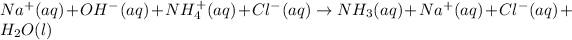
Cancelling the spectator ions from the above equation. Then the net ionic equation will be as follows.