Answer:
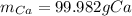
Step-by-step explanation:
Hello, in this case, considering that the undergoing chemical reaction is:
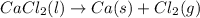
As we see a one-to-one molar relationship between calcium chloride and calcium, by stoichiometry and the given molar masses, the mass of calcium that would be formed is:
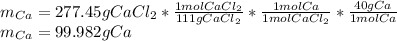
Best regards.