Answer:
The given reaction does not follow law of conservation of mass .
The Balanced reaction which follow law of conservation of mass is :
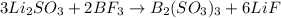
Step-by-step explanation:
LAW OF CONSERVATION OF MASS : Mass can neither created nor destroyed in a chemical reaction.
So, total number of atoms present in reactant should be equal to total number of elements present in the product.
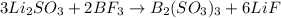
Here ,
total number of atoms present in reactant = total number of elements present in the product.
Li = 6
B = 2
F = 6
S = 3
O = 9