Answer:
a. Synthesis reaction
b. Single - Displacement
c. Decomposition reaction
d. Combustion Reaction
Step-by-step explanation:
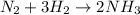
Synthesis reaction: Reaction in which two or more substance combine to give a single compound.
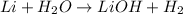
Single - Displacement : Reaction in which the more reactive element displaces the less reactive element.Here Li replace OH from water.
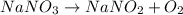
Decomposition reaction: Reaction in which a single reactant decompose to give two or more products.
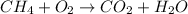
Combustion Reaction : IT is a redox reaction in which a substance is burned in the presence of oxygen to give carbon dioxide and water.