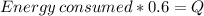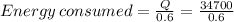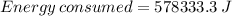Answer:
578333.3 joules are consumed too raise the temperature of a 50-kg body by 2°C
Step-by-step explanation:
Specific heat tells how much heat per unit of mass is needed to raise the temperature one degree Celsius. To find how much heat is needed to raise the temperature of an object a certain number of degrees we should use the equation:
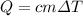 (1) with Q the heat needed, c the specific heat, m the mass of the object and
(1) with Q the heat needed, c the specific heat, m the mass of the object and
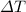 the change in temperature.
the change in temperature.
If we want to know how much heat is needed to raise the temperature of a 50 kg body by 2 degree Celsius, we only must use the numerical input on (1):
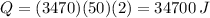
But because metabolism only can convert about 60% of energy to heat, we should give the metabolism more energy than 34700, we can find that value with the equation: