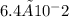Compete Question:
What mass of CDP (403 g mol−1) is in 10 mL of the buffered solution at the beginning of Experiment 1?
Passage: "16 mmol of CDP in 1 L of buffer"
Answer:
6.4 × 10-2 g
Step-by-step explanation:
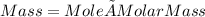
we are given from the question that 16 mmol of CDP is in 1 L of buffer
this mean that we have
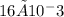 moles of CDP in 1 liter of buffer.
moles of CDP in 1 liter of buffer.
so the mass of CDP in one liter of buffer will be calculate as,
mass of CDP =
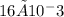 × 403g mol−1
× 403g mol−1
=
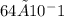
= 6.4 g/L
But because the question
asks us about the mass of CDP in 10 mL of solution, we will go further to calculate it like this:
6.4 g/L × 10 mL
6.4 g/L × 0.01 L =