Answer:
double displacement reaction
(Acid- base)
Step-by-step explanation:
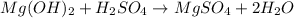
Double Displacement Reaction : The reaction in which the anion and cations of two different salts exchange their position to form new products.
Here ,
Mg2+ is exhanged by H+ to form H2O
SO4 2- by OH- to form H2O
H+ is exhanged by Mg+ to form MgSO4
OH- by SO4 (2-) to form MgSO4